UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):
(Exact name of registrant as specified in its charter)
|
|
|
|
|
|
|
(State or other jurisdiction of incorporation) |
|
(Commission File Number) |
|
(IRS Employer Identification No.)
|
|
|
|
|
|
|
|
(Address of Principal Executive Offices) |
|
|
|
(Zip Code) |
Registrant’s telephone number, including area code: (
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligations of the registrant under any of the following provisions:
|
|
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
|
|
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
|
|
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
|
|
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered |
|
|
|
The |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
|
Item 8.01 |
Other Events. |
Clinical Trial
On May 16, 2022, aTyr Pharma, Inc. (the “Company”), announced a Phase 3 study evaluating the efficacy and safety of its lead therapeutic candidate, efzofitimod (the non-proprietary name for ATYR1923), in patients with pulmonary sarcoidosis. The study, which will be known as EFZO-FIT™, is expected to initiate in the third quarter of 2022.
The EFZO-FIT™ study is a global Phase 3 randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of efzofitimod in patients with pulmonary sarcoidosis. This will be a 52-week study consisting of three parallel cohorts randomized equally to either 3.0 mg/kg or 5.0 mg/kg of efzofitimod or placebo dosed intravenously once a month for a total of 12 doses. The study intends to enroll 264 subjects with pulmonary sarcoidosis at multiple centers in North America, Europe and Japan. The trial design will incorporate a forced steroid taper. The primary endpoint of the study is steroid reduction. Secondary endpoints include measures of lung function and sarcoidosis symptoms.
Efzofitimod is a first-in-class immunomodulator that downregulates innate and adaptive immune responses in uncontrolled inflammatory diseases states via selective modulation of neuropilin-2. Clinical proof-of-concept was recently established for efzofitimod in a Phase 1b/2a study in patients with pulmonary sarcoidosis, a major form of interstitial lung disease (ILD).
Poster Presentations
On May 17, 2022, the Company announced that clinical data for efzofitimod will be presented in two posters on May 17, 2022 from 11:15AM – 1:15PM PT at the American Thoracic Society (ATS) 2022 International Conference in San Francisco, CA.
Details of the poster presentations are set forth below. The corresponding abstracts are available for review online on the conference website. The posters will be available on the Investor Relations section of the Company’s website at www.atyrpharma.com once presented.
Title: Safety and Efficacy ATYR1923, a Novel Immunomodulator for Pulmonary Sarcoidosis: Results of a Phase 1b/2a Randomized Placebo-Controlled Trial
Abstract Number: 3932
Poster Number: P559
Poster Session: Inflammatory Modulation in Sarcoidosis, Lung Transplant, and Other Diseases
Date and Time: Tuesday, May 17, 2022 from 11:15AM – 1:15PM PT
Location: Area G, Hall F (North Building, Exhibition Level), Moscone Center
The poster presents findings from a Phase 1b/2a randomized, double-blind, placebo-controlled study of efzofitimod in patients with pulmonary sarcoidosis. Monthly dosing of efzofitimod was safe and well tolerated. There was a dose-dependent improvement in efficacy parameters, including corticosteroid (CS) taper, percent-predicted forced vital capacity (FVCPP), and patient reported outcomes. All efzofitimod treatment groups had lower (CS) use at week 24 compared to placebo, with the largest difference observed in the 5.0 mg/kg treatment group, where three patients were able to taper off CS completely and maintain that taper through the completion of the study. The two higher doses of efzofitimod, 3.0 mg/kg and 5.0 mg/kg, resulted in improvements in FVCPP and percent-predicted diffusing capacity of the lungs for carbon monoxide (DLCOPP) through week 24 compared to placebo. Clinically meaningful and statistically significant improvements at week 24 were observed for key symptom measures in the 5.0 mg/kg treatment group. In small studies such as this, which was not powered for statistical significance, dose dependent improvements are strong evidence for efficacy.
Title: ATYR1923 Treatment Reduces Pro-Inflammatory Serum Biomarkers in Pulmonary Sarcoidosis Patients
Abstract Number: 3933
Poster Number: P560
Poster Session: Inflammatory Modulation in Sarcoidosis, Lung Transplant, and Other Diseases
Date and Time: Tuesday, May 17, 2022 from 11:15AM – 1:15PM PT
Location: Area G, Hall F (North Building, Exhibition Level), Moscone Center
The poster presents clinical biomarker findings from a Phase 1b/2a randomized, double-blind, placebo-controlled study of efzofitimod in patients with pulmonary sarcoidosis. Efzofitimod demonstrated dose dependent control of inflammatory and sarcoidosis disease biomarkers over 24 weeks in the context of a CS taper. The affected inflammatory biomarkers, including IFN-γ, IL-6, IP-10, MCP-1 and TNFa, and key markers of sarcoidosis, including IL-2Ra, SAA, ACE enzyme and ACE protein, are key drivers of sarcoidosis and other ILD and consistent with results from preclinical animal models and a Phase 2 study of efzofitimod in hospitalized COVID-19 pneumonia patients. These results are the first demonstration of efzofitimod’s anti-inflammatory mechanism in patients with pulmonary
2
sarcoidosis. These analyses were exploratory and not adjusted for multiplicity to control for false positive results and will need to be confirmed in a larger study.
A corporate presentation regarding the Phase 3 study design and the Phase 1b/2a clinical study data is attached hereto as Exhibit 99.1.
|
Item 9.01 |
Financial Statements and Exhibits. |
(d) Exhibits
|
Exhibit No. |
|
Description |
|
|
|
|
|
99.1 |
|
|
|
|
|
|
|
104 |
|
Cover Page Interactive Data File (embedded within the Inline XBRL document) |
3
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
ATYR PHARMA, INC. |
|
|
|
|
|
|
|
|
|
|
|
By: |
/s/ Jill M. Broadfoot |
|
|
|
Jill M. Broadfoot |
|
|
|
Chief Financial Officer |
|
|
|
|
|
Date: May 17, 2022 |
|
|
4

A New Path to Medicine Company Reception May 16, 2022 Exhibit 99.1

Forward Looking Statements The following slides and any accompanying oral presentation contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and other federal securities laws. The use of words such as “may,” “might,” “will,” “should,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “project,” “intend,” “future,” “potential,” “opportunity,” or “continue,” and other similar expressions are intended to identify forward-looking statements. For example, all statements regarding: the potential therapeutic benefits of proteins derived from tRNA synthetase genes and our product candidates, including efzofitimod and ATYR2810, and development programs, including our NRP2 antibody program and our tRNA synthetase program; the ability to successfully advance our product candidates and undertake certain development activities (such as the initiation of clinical trials, clinical trial enrollment, the conduct of clinical trials and announcement of clinical results) and accomplish certain development goals, and the timing of such events; the potential market opportunity for our product candidates; our ability to receive regulatory approvals for, and commercialize, our product candidates; our ability to identify and discover additional product candidates; potential activities and payments under collaboration agreements; and the ability of our intellectual property portfolio to provide protection are forward-looking statements. All forward-looking statements are based on estimates and assumptions by our management that, although we believe to be reasonable, are inherently uncertain. All forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially from those that we expected. These risks, uncertainties and other factors are more fully described in our filings with the U.S. Securities and Exchange Commission, including our Annual Report on Form 10-K, our Quarterly Reports on Form 10-Q, and in our other filings. The forward-looking statements in this presentation speak only as of the date of this presentation and neither we nor any other person assume responsibility for the accuracy and completeness of any forward-looking statement. We undertake no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise, except as required by law. We own various U.S. federal trademark applications and unregistered trademarks, including our company name. All other trademarks or trade names referred to in this presentation are the property of their respective owners. Solely for convenience, the trademarks and trade names in this presentation are referred to without the symbols ® and ™, but such references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto. 2

Efzofitimod: A Novel Mechanism to Treat Lung Inflammation and Fibrosis 3 Representative histology showing immune cell infiltrate and collagen content in a rat model of bleomycin induced lung fibrosis presented at the ATS annual meeting 2018. Placebo treated Efzofitimod treated Healthy lung Inflammation Fibrosis
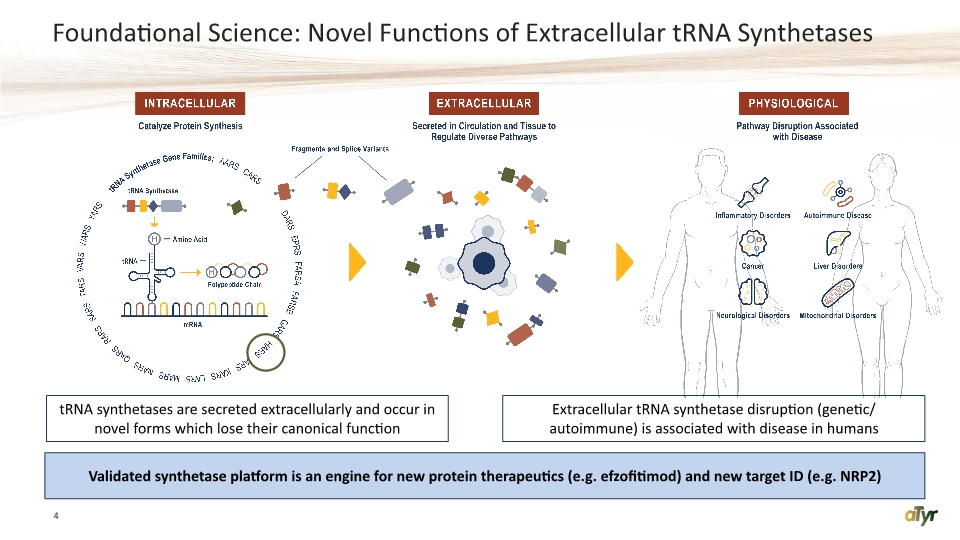
Foundational Science: Novel Functions of Extracellular tRNA Synthetases 4 tRNA synthetases are secreted extracellularly and occur in novel forms which lose their canonical function Extracellular tRNA synthetase disruption (genetic/ autoimmune) is associated with disease in humans Validated synthetase platform is an engine for new protein therapeutics (e.g. efzofitimod) and new target ID (e.g. NRP2)

Efzofitimod (ATYR1923, KRP-R120) A Novel Immunomodulator for Fibrotic Lung Disease

Efzofitimod: First-in-Class Therapy for Fibrotic Lung Disease 6 MOA = mechanism of action

7 Efzofitimod Therapeutic Goal: Restore Immune Balance and Prevent Fibrosis
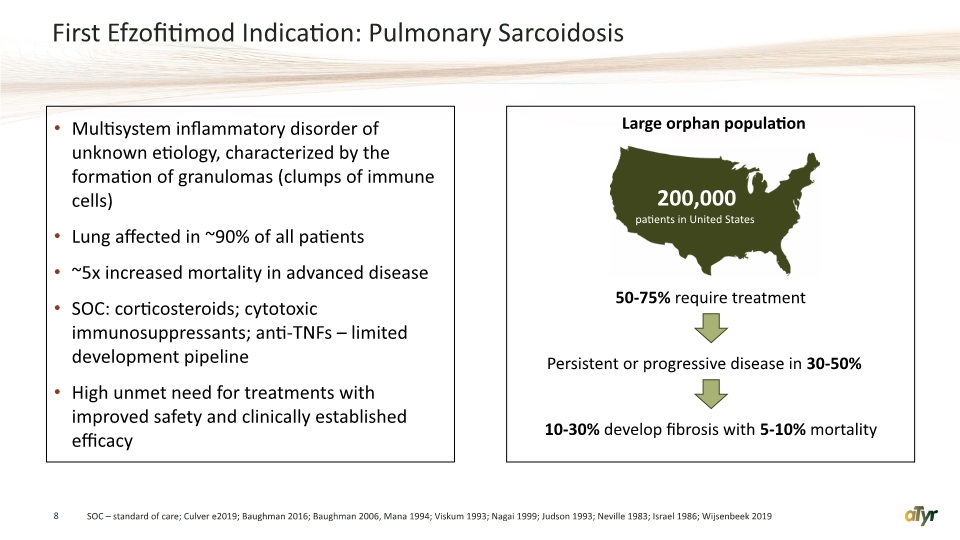
First Efzofitimod Indication: Pulmonary Sarcoidosis Multisystem inflammatory disorder of unknown etiology, characterized by the formation of granulomas (clumps of immune cells) Lung affected in ~90% of all patients ~5x increased mortality in advanced disease SOC: corticosteroids; cytotoxic immunosuppressants; anti-TNFs – limited development pipeline High unmet need for treatments with improved safety and clinically established efficacy 8 SOC – standard of care; Culver e2019; Baughman 2016; Baughman 2006, Mana 1994; Viskum 1993; Nagai 1999; Judson 1993; Neville 1983; Israel 1986; Wijsenbeek 2019 Persistent or progressive disease in 30-50% 50-75% require treatment Large orphan population 10-30% develop fibrosis with 5-10% mortality

Efzofitimod (ATYR1923, KRP-R120) Results from Phase 1b/2a Study in Pulmonary Sarcoidosis Peter H. S. Sporn, M.D. Professor of Medicine (Pulmonary and Critical Care); Cell and Developmental Biology; and Medical Education Northwestern University Feinberg School of Medicine
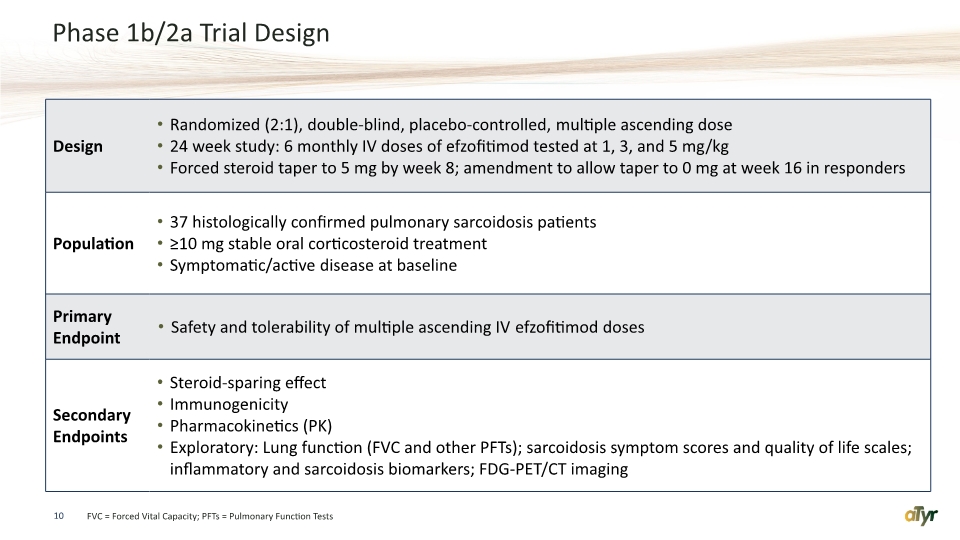
Phase 1b/2a Trial Design 10 FVC = Forced Vital Capacity; PFTs = Pulmonary Function Tests

Phase 1b/2a Inclusion / Exclusion Criteria Inclusion Histologically proven diagnosis of pulmonary sarcoidosis Stable treatment with 10 -25 mg/day oral corticosteroid oral immunomodulator allowed Symptomatic/active disease at baseline FVC ≥ 50% percent predicted MRC Dyspnea Scale score (>= 1) Exclusion Disease consistent with Lofgren's syndrome Treatment with biological immunomodulator such as tumor necrosis factor-alpha inhibitors Clinically significant cardiac, neurological, gastrointestinal, and/or renal sarcoidosis PH requiring vasodilator treatment 11
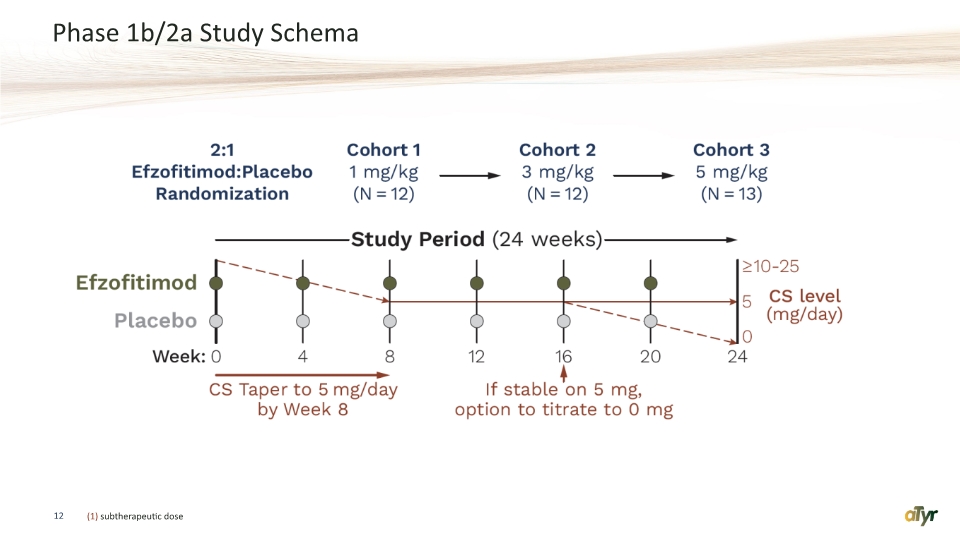
Phase 1b/2a Study Schema 12 (1) subtherapeutic dose
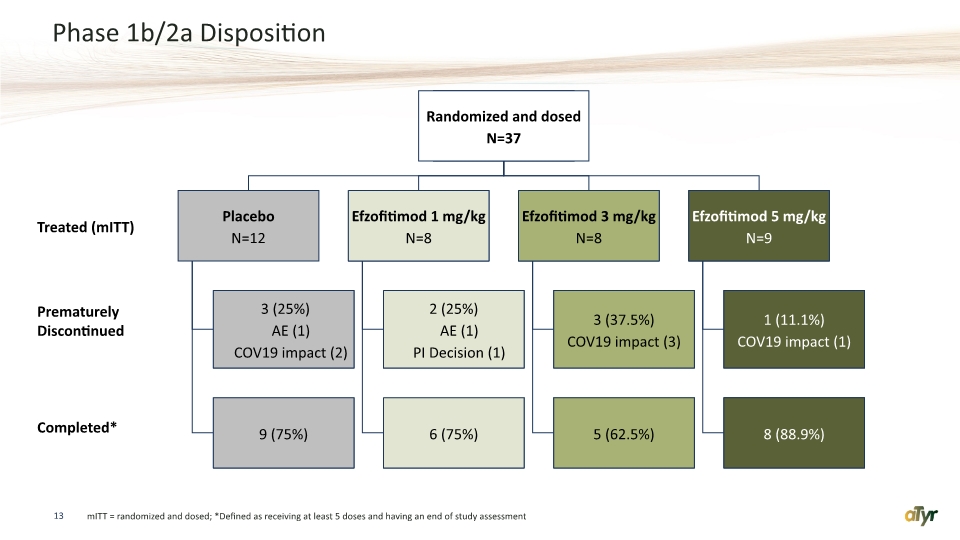
Phase 1b/2a Disposition mITT = randomized and dosed; *Defined as receiving at least 5 doses and having an end of study assessment Treated (mITT) Prematurely Discontinued Completed* Table 14.1.1.1 23Aug2021 13
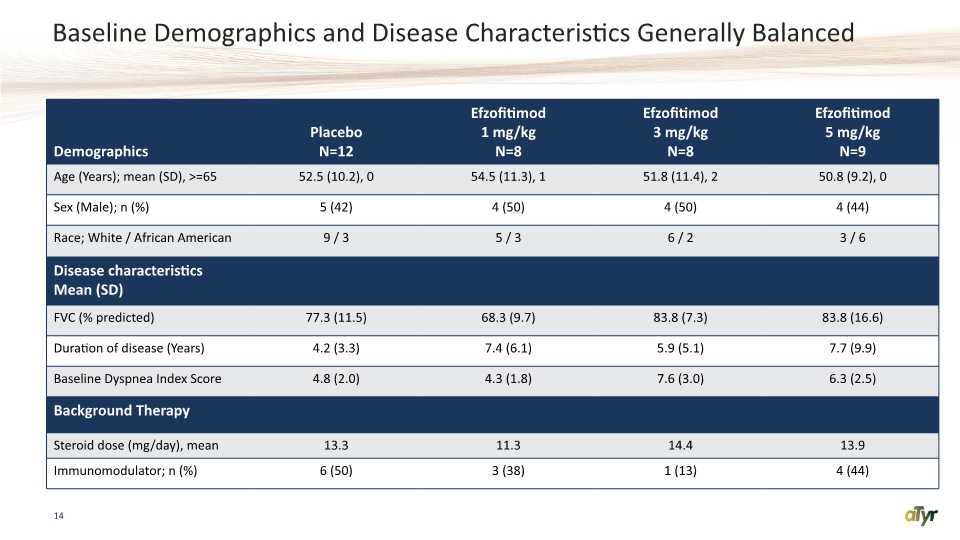
Baseline Demographics and Disease Characteristics Generally Balanced 14

Monthly Dosing of Efzofitimod was Safe and Well-Tolerated No new or unexpected findings with repeat dosing No dose-response relationship observed Most frequent AEs were consistent with underlying disease: Cough, fatigue, wheezing No signal of immunogenicity No drug related SAEs No deaths 15 Safety population

Dose-dependent Reduction in Steroid Utilization Compared with Placebo 58% overall steroid reduction from baseline and 22% relative reduction compared to placebo in steroid utilization post taper in the 5 mg/kg treatment group 33% of patients able to taper off of steroids completely in 5 mg/kg treatment group while controlling disease symptoms 16 D51-EOS; adjusted mean refers to least squares mean from ANCOVA adjusting for baseline steroid use
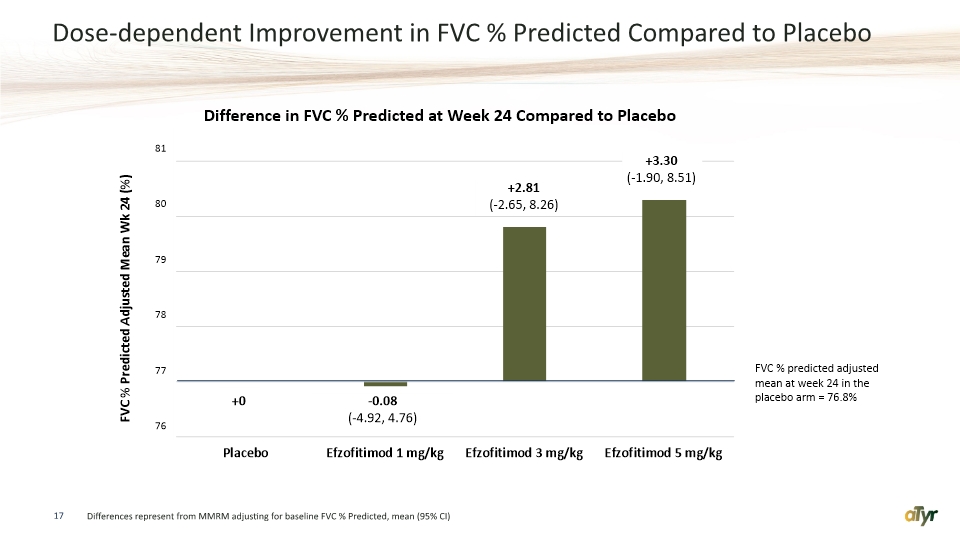
Dose-dependent Improvement in FVC % Predicted Compared to Placebo 17 Differences represent from MMRM adjusting for baseline FVC % Predicted, mean (95% CI)

Higher Efzofitimod Doses Show Trends of Improvement in FVC % Predicted 18

Dose-dependent Improvements in PFTs Over Time Compared with Placebo 19 PFTs = Pulmonary Function Tests; CFB = Change From Baseline Where is week 24 data?

Dose-dependent Symptom Improvements Compared with Placebo 20 *p<0.05 on difference between adjusted means from MMRM; Pbo = Placebo MCIDs: TDI - Witek 2003; LCQ – Raj 2009; FAS - de Kleijin 2011 (Negative score is better for Fatigue); KSQ Lung – Baughman 2021; KSQ Lung – Baughman 2021 = clinically meaningful improvement based on published MCID

Dose-dependent Control of Key Disease and Inflammatory Biomarkers 21 Graphs represent fold change at Week 24 compared to Baseline IFNg IP-10 / CXCL10 MCP-1 / CCL2 IL-2Ra

Phase 1b/2a Data Supports Proof-of-Concept and Clinical Advancement Efzofitimod was safe and well-tolerated Strong suggestion of efficacy: Dose-response and consistent positive trends across key efficacy endpoints and multiple analysis populations Clinically meaningful symptom improvements in dyspnea, cough, fatigue and King’s Sarcoidosis scores Dose dependent control of inflammatory biomarkers confirms anti-inflammatory effect 22

Efzofitimod (ATYR1923, KRP-R120) Phase 3 EFZO-FIT™ Study Robert P. Baughman, M.D. Emeritus Professor of Medicine University of Cincinnati

Dose-dependent Reduction in Steroid Utilization Compared with Placebo 58% overall steroid reduction from baseline and 22% relative reduction compared to placebo in steroid utilization post taper in the 5 mg/kg treatment group 33% of patients able to taper off of steroids completely in 5 mg/kg treatment group while controlling disease symptoms 24 D51-EOS; adjusted mean refers to least squares mean from ANCOVA adjusting for baseline steroid use

Dose-dependent Symptom Improvements Compared with Placebo 25 *p<0.05 on difference between adjusted means from MMRM; Pbo = Placebo MCIDs: TDI - Witek 2003; LCQ – Raj 2009; FAS - de Kleijin 2011 (Negative score is better for Fatigue); KSQ Lung – Baughman 2021; KSQ Lung – Baughman 2021 = clinically meaningful improvement based on published MCID

EFZO-FITTM : Phase 3 Study of Efzofitimod in Pulmonary Sarcoidosis 26

Trial Design 27 Still under discussion with Kyorin clinical team
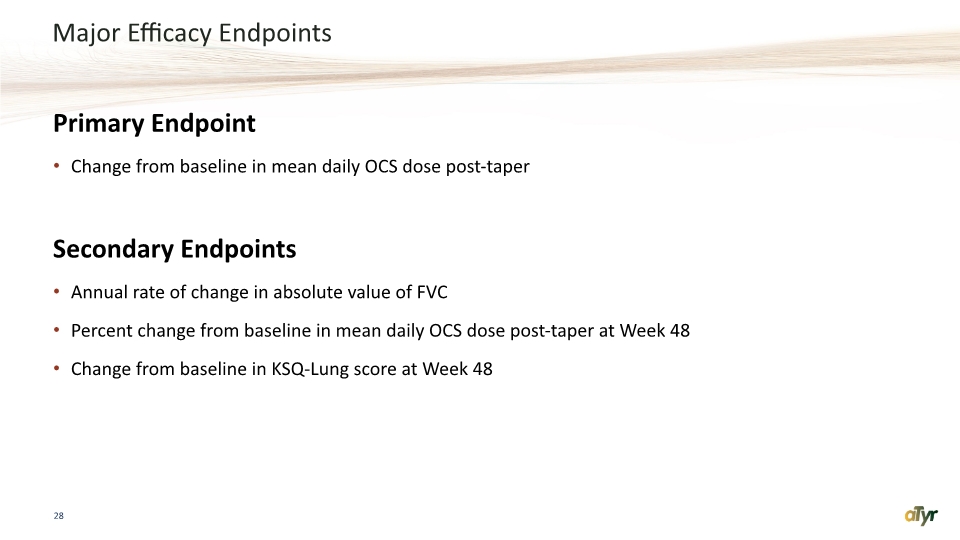
Major Efficacy Endpoints Primary Endpoint Change from baseline in mean daily OCS dose post-taper Secondary Endpoints Annual rate of change in absolute value of FVC Percent change from baseline in mean daily OCS dose post-taper at Week 48 Change from baseline in KSQ-Lung score at Week 48 28

Key Inclusion / Exclusion Criteria Inclusion Adults ages 18-75, inclusive Diagnosis of pulmonary sarcoidosis for ≥6 months Requiring stable treatment with ≥7.5 but ≤25 mg/day oral corticosteroids Medical Research Council (MRC) Dyspnea Scale ≥ 1 KSQ Lung Score ≤ 70 Exclusion Extent of fibrosis > 20% Forced Vital Capacity < 50% Treatment > 1 oral immunomodulator Clinically significant pulmonary hypertension Patients with cardiac/neuro/renal sarcoidosis Treatment with biological immunomodulators 29
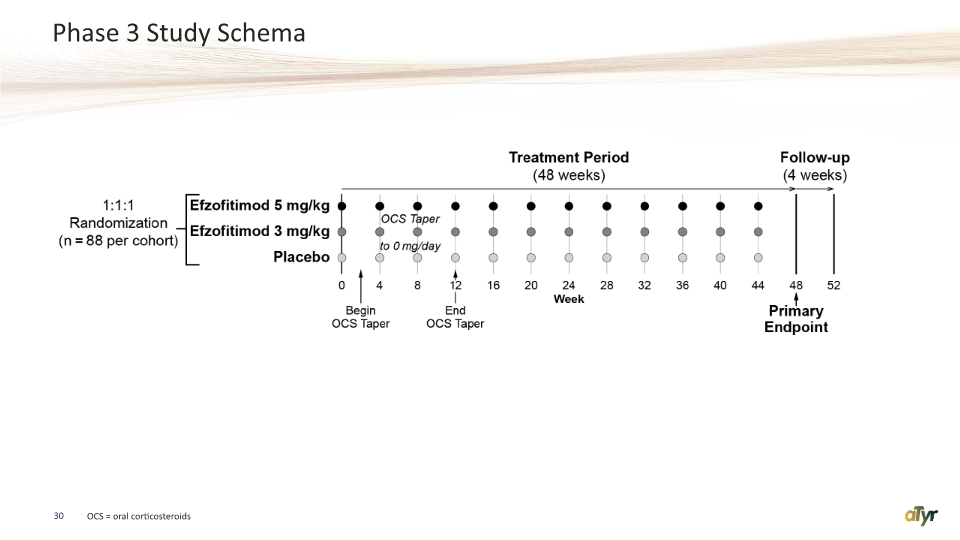
Phase 3 Study Schema 30 OCS = oral corticosteroids

Multi-center Trial with Sites in North America, Europe and Japan 31 Anticipating 60-80 centers in 10 countries

Thank You
