UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): January 8, 2021
ATYR PHARMA, INC.
(Exact name of registrant as specified in its charter)
|
Delaware |
|
001-37378 |
|
20-3435077 |
|
(State or other jurisdiction of incorporation) |
|
(Commission File Number) |
|
(IRS Employer Identification No.)
|
|
3545 John Hopkins Court, Suite #250 San Diego |
|
|
|
92121 |
|
(Address of Principal Executive Offices) |
|
|
|
(Zip Code) |
Registrant’s telephone number, including area code: (858) 731-8389
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligations of the registrant under any of the following provisions:
|
☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
|
☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
|
☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
|
☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered |
|
Common Stock, par value $0.001 per share |
LIFE |
The Nasdaq Capital Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 or Rule 12b-2 of the Securities Exchange Act of 1934.
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☒
Item 7.01 Regulation FD Disclosure.
aTyr Pharma, Inc. (the “Company”) intends to use an investor presentation to conduct meetings with investors, stockholders and analysts and at investor conferences. The Company intends to place this investor presentation on its website. A copy of the presentation materials is attached hereto as Exhibit 99.1. The Company does not undertake to update the presentation materials.
The information under this Item 7.01, including Exhibit 99.1, is being furnished and shall not be deemed “filed” for the purposes of Section 18 of the Securities and Exchange Act of 1934, as amended, or the Exchange Act, or otherwise subject to the liabilities of that section, nor shall they be deemed incorporated by reference into any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such filing
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
|
Exhibit No. |
|
Description |
|
|
|
|
|
99.1 |
|
Corporate Presentation Materials of aTyr Pharma, Inc. dated January 2021 |
|
|
|
|
|
|
|
|
|
|
|
|
2
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
ATYR PHARMA, INC. |
|
|
|
|
|
|
|
|
|
|
|
By: |
/s/ Jill M. Broadfoot |
|
|
|
Jill M. Broadfoot |
|
|
|
Chief Financial Officer |
|
|
|
|
|
Date: January 8, 2021 |
|
|
3

A New Path to Medicine January 2021 Exhibit 99.1

Forward Looking Statements The following slides and any accompanying oral presentation contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and other federal securities laws. The use of words such as “may,” “might,” “will,” “should,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “project,” “intend,” “future,” “potential,” “opportunity,” or “continue,” and other similar expressions are intended to identify forward-looking statements. For example, all statements regarding: the potential therapeutic benefits of proteins derived from tRNA synthetase genes and our product candidates, including ATYR1923 and ATYR2810, and development programs, including our NRP2 antibody program and our tRNA synthetase program; the ability to successfully advance our product candidates and undertake certain development activities (such as the initiation of clinical trials, clinical trials enrollment, the conduct of clinical trials and announcement of clinical results) and accomplish certain development goals, and the timing of such events; the potential market opportunity for our product candidates; our ability to receive regulatory approvals for, and commercialize, our product candidates; our ability to identify and discover additional product candidates; potential activities and payments under collaboration agreements; and the ability of our intellectual property portfolio to provide protection are forward-looking statements. All forward-looking statements are based on estimates and assumptions by our management that, although we believe to be reasonable, are inherently uncertain. All forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially from those that we expected. These risks, uncertainties and other factors are more fully described in our filings with the U.S. Securities and Exchange Commission, including our Annual Report on Form 10-K, our Quarterly Reports on Form 10-Q, and in our other filings. The forward-looking statements in this presentation speak only as of the date of this presentation and neither we nor any other person assume responsibility for the accuracy and completeness of any forward-looking statement. We undertake no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise, except as required by law. We own various U.S. federal trademark applications and unregistered trademarks, including our company name. All other trademarks or trade names referred to in this presentation are the property of their respective owners. Solely for convenience, the trademarks and trade names in this presentation are referred to without the symbols ® and ™, but such references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto. 2

aTyr: A New Path to Medicine 3
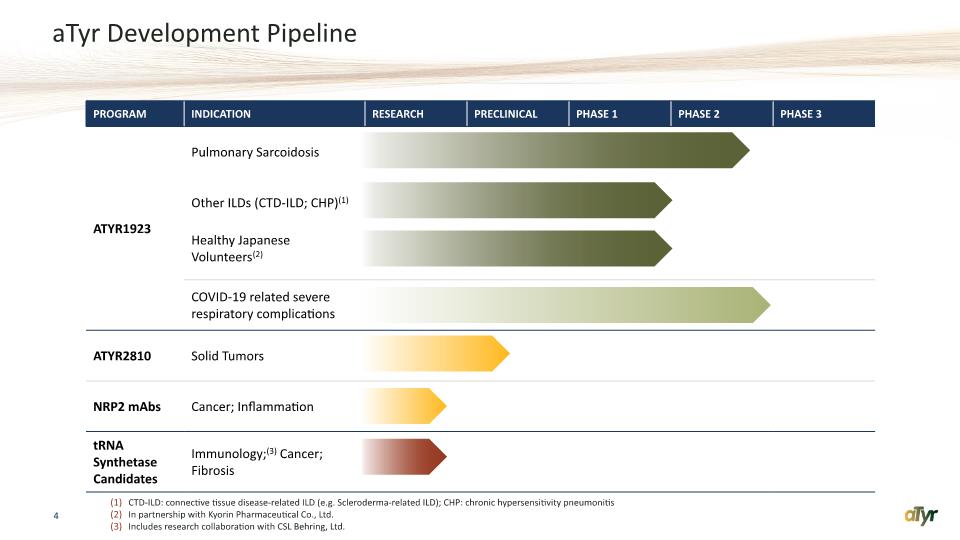
aTyr Development Pipeline 4 CTD-ILD: connective tissue disease-related ILD (e.g. Scleroderma-related ILD); CHP: chronic hypersensitivity pneumonitis In partnership with Kyorin Pharmaceutical Co., Ltd. Includes research collaboration with CSL Behring, Ltd.

tRNA Synthetases May Have Novel Functions Extracellularly 5

ATYR1923 A Novel Immunomodulator for Inflammatory Lung Disease
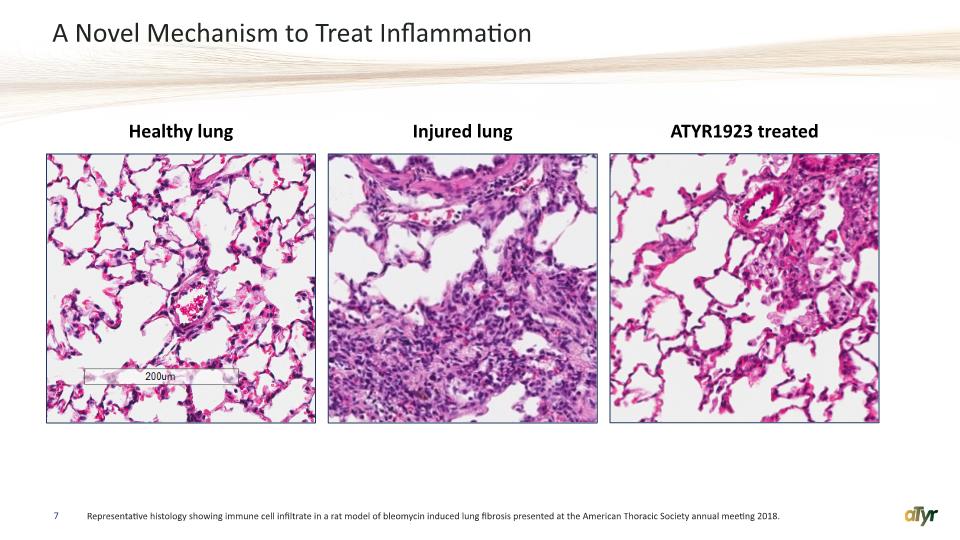
A Novel Mechanism to Treat Inflammation 7 Representative histology showing immune cell infiltrate in a rat model of bleomycin induced lung fibrosis presented at the American Thoracic Society annual meeting 2018. Injured lung ATYR1923 treated Healthy lung
ATYR1923: Early Data Supports Therapeutic Potential in Immune-Mediated Lung Disease Fc fusion protein, based on lung-enriched histidyl-tRNA synthetase (HARS) fragment, recombinantly expressed in E. coli Receptor screen identified selective binding to NRP2, a cell surface receptor upregulated on key immune cells during inflammation and is enriched in inflamed lung tissue NRP2expression is detected in granulomas associated with human sarcoidosis of the lung and skin Lung tissue from patients who died from COVID-19 related respiratory failure shows increased NRP2 Downregulates inflammatory and pro-fibrotic cytokine and chemokine levels as well as histological inflammation and fibrosis in pre-clinical models Phase 1 study in healthy volunteers PK supports with once-monthly intravenous dosing Well tolerated in patients and subjects dosed to date with exposure up to 24 weeks 8

Demonstrated Effect in Animal Lung Injury Models 9 Data available at https://www.atyrpharma.com/our-science/publications/ Fibrosis Inflammation Bleomycin-Induced Lung Fibrosis (IPF) P. acnes (Sarcoidosis) Sclerodermatous GvHD (SSc-ILD) SKG mice (RA-ILD) S. Rectivirgula (CHP) ↓ inflammation / fibrosis ↓ fibrosis ↓ immune infiltrates ↓ tissue cytokines/chemokines and enzymes ↓ pro-fibrotic proteins Consistent downregulation of pro-inflammatory cytokines including IL-6, MCP-1, and IFN-γ

ATYR1923 Mechanism of Action in Inflammatory Lung Disease 10

ATYR1923 Interstitial Lung Disease

ILDs Share Common Immune Pathology Leading to Fibrosis 12 Slide adapted from Dr. Steven Nathan, Medical Director, Advanced Lung Disease and Transplant Program at Inova Fairfax Hospital, Falls Church, VA Fibrosis Pulmonary Sarcoidosis Connective Tissue Disease related – ILD (CTD-ILD) Chronic Hypersensitivity Pneumonitis (CHP) Idiopathic Pulmonary Fibrosis (IPF) Inflammation ILDs lie on a spectrum of inflammation and fibrosis, but all share an immune pathology Progression to fibrosis is a key driver of morbidity and mortality By targeting aberrant immune response driving fibrosis, ATYR1923 has potential to improve outcomes in multiple ILDs

Market Opportunity in Inflammatory Interstitial Lung Disease 13 Lederer, Martinez. NEJM 2018 All ILDs individually have potential for orphan status aTyr estimates for ATYR1923 in Pulmonary Sarcoidosis, CHP, CTD-ILD; excludes IPF >200 types of ILD: 4 major types comprise 80% of patients Limited standard of care with substantial morbidity and mortality aTyr focused on 3 most inflammatory types: ~500-600k US patients(2); ~3m globally $2-3b global market opportunity(3) Relative Distribution of ILDs in the USA(1) aTyr focus

First ATYR1923 Indication: Pulmonary Sarcoidosis Inflammatory disease of unknown etiology characterized by the formation of granulomas (clumps of immune cells) T cell driven: CD4+ (Th1 / Th17) Pulmonary sarcoidosis occurs in ~90% of all sarcoidosis patients Treatment options are limited with associated toxicity: Corticosteroids, antimetabolite immunosuppressants, TNF inhibitors 14 Culver et al. BMJ 2019; Baughman ATS Annals 2016

Phase 1b/2a Study in Pulmonary Sarcoidosis Ongoing 15 Target enrollment completed Data expected Q3 2021

Phase 1b/2a Pulmonary Sarcoidosis Study Schema 16 (1) subtherapeutic dose 0 4 8 12 16 20 24 24-week Study Period Week Each Cohort: Dose Escalation Review after 6 patients have received ≥3 doses of study drug ATYR1923 Placebo 5 mg OCS(1) Steroid taper every 2 weeks Primary endpoint If stable on 5 mg, option to completely titrate off steroids ≥10 mg OCS Steroid dose

ATYR1923 Japan Collaboration Kyorin Overview Founded: 1923 Focus: Respiratory, ENT, Urology 1600 employees: incl. 350 in R&D; 750 sales reps covering top respiratory centers in Japan Sales: ~$1b USD Market cap: $1.2b USD (4569:JP TSE) Key Terms Scope: ATYR1923; Japan; ILD Upfront payment: $8m Development, regulatory and commercial milestones: $167m Tiered sales royalties into double digits Kyorin to fund all development and commercial activities in Japan 17 Currently conducting Phase 1 study to evaluate the safety, pharmacokinetics and immunogenicity in Japanese healthy volunteers

ATYR1923 COVID-19 Related Severe Respiratory Complications

Phase 2 Study in COVID-19 Related Severe Respiratory Complications 19 Topline data reported January 2021 Full data set, including biomarker analysis, expected Q1 2021

Highlights of Topline Results for Safety and Key Recovery Metrics Study Met Primary Safety Endpoint in Moderate to Severe Hospitalized Patients ATYR1923 was generally safe and well-tolerated in both the 1.0 and 3.0 mg/kg treatment groups Adverse events were mostly mild or moderate in severity and there were no drug-related SAEs Preliminary Signal of Activity Seen in 3.0 mg/kg Cohort(1) 3.0 mg/kg cohort experienced a median time to recovery of 5.5 days (95% CI: 3,6) compared to 6 days (95% CI: 2,12) in placebo group 83% vs 56% of patients achieved recovery by day 6 in the 3.0 mg/kg ATYR1923 and placebo groups, respectively 20 (1) Study was not powered for statistical significance

Key Insights from Preliminary Demographics and Baseline Characteristics Demographics and baseline disease characteristics were largely balanced except for some key risk factors which were disproportionately randomized to ATYR1923 groups: More patients over the age of 65 More patients with severe hypoxia More baseline comorbidities and more patients with multiple baseline comorbidities Imbalances suggest a sicker patient population in ATYR1923 treatment groups and may have contributed to an overperformance in the placebo arm All patients in the study received remdesivir and/or dexamethasone 21

NRP2 Antibodies Regulating Diverse Disease Pathways

NRP2: A Novel Therapeutic Target NRP2 is a potentially novel drug target for cancer and inflammatory disorders Acts as a co-receptor for VEGF, semaphorins and certain chemokines (e.g., CCL19) Highly expressed on certain tumors and upregulated on immune cells during inflammation Tumor expression is associated with worse outcomes in many cancers aTyr has developed a panel of antibodies to selectively target different NRP2 epitopes for diverse therapeutic applications aTyr anti-NRP2 monoclonal antibodies exhibit superior specificity and sensitivity compared to commercially available antibodies 23

aTyr is Developing Humanized NRP2 Antibodies Targeting Diverse Pathways 24 (1) Distinct from ATYR1923 interaction with NRP2 SEMA3F VEGF Dimerization domain a1 a2 b1 b2 c ATYR2810 CCR7 Ligand Blockers a-NRP2-14 aTyr lead antibodies(1)

ATYR2810: Lead Anti-Neuropilin-2 Antibody IND Candidate for Cancer ATYR2810 is a humanized monoclonal antibody that specifically and functionally blocks the interaction between NRP2 and VEGF The role of NRP2 and VEGF signaling in the tumor microenvironment and its importance in the progression of certain aggressive cancers is becoming increasingly validated Preclinical data in models of triple-negative breast cancer suggest that ATYR2810 could potentially be effective in certain aggressive solid tumors (1) Blocks VEGF-C binding to NRP2 Shows tumor inhibitory effects Increases sensitivity to chemotherapy 25 (1) Domain-Specific Antibodies to Neuropilin 2 Implicate VEGF-C and not Semaforin 3F in Breast Cancer Stem Cell Function. American Association for Cancer Research, 2020

Early Pre-clinical Data Support Development in Oncology 26 AACR Virtual Meeting II – June 22-24, 2020; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0..0001 Increases Sensitivity to Chemotherapy in Triple-Negative Breast Cancer Model Blocks VEGF binding to NRP2

tRNA Synthetases A Potential New Therapeutic Protein Class

tRNA Synthetases: A Potential New Therapeutic Protein Class aTyr owns IP covering proteins derived from all 20 tRNA synthetase gene families Lead program ATYR1923 is based on a naturally occurring splice variant of one of these families, histidyl-tRNA synthetase (HARS) tRNA synthetases are secreted extracellularly and occur in novel forms which lose their canonical function Extracellular tRNA synthetase disruption (genetic / autoimmune) is associated with disease in humans aTyr working with leading biopharmaceutical company CSL Behring on identifying new IND candidates for immunological disorders in up to four tRNA synthetases from aTyr’s pipeline (non-HARS derived) Recent discovery of new receptor targets for two tRNA synthetases in cancer and inflammation 28

A New Path to Medicine

aTyr: A New Path to Medicine Platform of proprietary new biology Phase 2 clinical program: ATYR1923 Novel MOA for inflammatory lung disease Demonstrated effect in multiple animal lung injury models Phase 1b/2a clinical study in pulmonary sarcoidosis completed enrollment in US – positive interim safety data reported Dec 2019 Kyorin collaboration for ILD in Japan with total deal value up to $175m; conducting Phase 1 study Phase 2 trial in COVID-19 patients with severe respiratory complications completed– positive topline results reported January 2021 Preclinical program: ATYR2810 Lead anti-NRP2 antibody IND candidate for cancer shows anti-tumor effects in triple-negative breast cancer model Discovery stage programs in cancer, inflammation and immunology NRP2 antibody research program for distinct therapeutic applications New receptor targets identified for two tRNA synthetases in cancer and inflammation Cash, cash equivalents, and investments at $36.1m as of September 30, 2020 30

Upcoming Catalysts 31

Thank You
